The chemistry at work
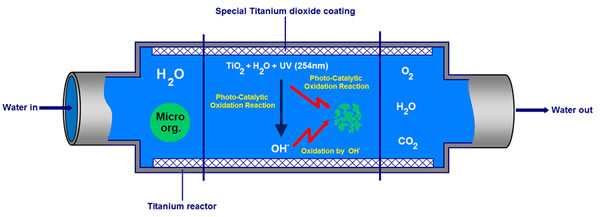
The Titanium AOP System is a physical process that works by exploiting the photo-catalytic effect created when high energy Ultra Violet UV light (at 254 nm) strikes Titanium Dioxide (TiO2) in the presence of water. The high energy UV excites the surface of the TiO2 at the water interface resulting in a photo-catalytic oxidation reaction. This oxidising reaction is one of the strongest known and is at the heart of the Titanium AOP Unit.
The photo-catalytic oxidation reaction has two effects. Primarily it will oxidise and destroy any contaminate or pollutant present where the reaction occurs. In the case of the Titanium AOP this is the entire surface area of the reactor that is contact with the water. The second reaction oxidises water to generate a proliferation of Hydroxyl Radicals that in turn will also oxidise any contaminate or pollutant present.
We can break down these two key Oxidising reactions as follows:
Step One (Reaction 1): Photo-Catalytic Oxidation.
This occurs at the surface when UV at 254nm hits and excites TiO2 in the presence of water. This reaction has two effects:
- It will oxidise (and therefore destroy) any pollutant present at the reactor surface.
- Oxidise water to Hydroxyl Radicals OH . The benefit of creating these Hydroxyl Radicals OH is detailed in step 2 below.

Step Two (Reaction 2): Oxidation by Hydroxyl Radicals
The Hydroxyl Radicals generated as a result of reaction 1 are dispersed throughout the reactors water chamber due to them being extremely mobile. The Hydroxyl Radical has very high oxidation strength, a short life and is very reactive.
They are exceptionally strong oxidising agents and oxidise any organic pollutant indiscriminately.
What is a Hydroxyl Radical?
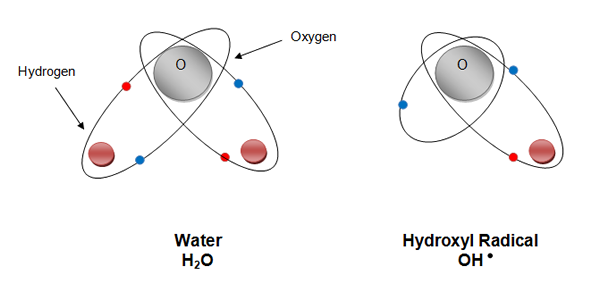
In essence it is a water molecule with a hydrogen atom removed.
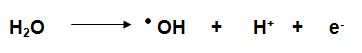
They contain an unpaired electron which makes them very reactive and highly unstable. Due to their extreme reactivity Hydroxyl Radicals only exist for milliseconds before reverting back to water. They are exceptionally strong oxidising agents and oxidise any organic pollutant indiscriminately.
Summary
Due to the short life of both reactions the process takes place entirely within the AOP reactor. The AOP oxidises all organic material living or dead to Carbon Dioxide (CO2), Oxygen (O2) and Water (H2O). Other than destroying such pollutants the Titanium AOP has no effect on the basic water chemistry. The process is truly catalytic as the TiO2 is not sacrificed or consumed and therefore the AOP reactor carries a 10 year warranty.